Avidin
Avidin: How to be Innovative with a Binding Protein
e-Proteins is the only native Avidin producer that has managed to reach 100% purity. This high quality is crucial when Avidin has usage in different applications and also has to offer high performances of the binding. Whether you want to increase the purification capacity, increase the detection signal or reduce the non-specific binding, the purity of the product is the principal element to ensure performance, reliability and repeatability.
100% Avidin Purity: The Start of all Cutting Edge Derivatives and Conjugates
Avidin is basically an egg protein that has many different functions. Initially, in-vitro diagnosis kits used it, but now other fields such as cell culture are also using it. Avidin helps neutralize biotins and improves the purification process on a chromatography column. The improvement of the Avidin production process allows it to retain 100% purity level successfully.
Avidin has been of interest to some universities and organizations for several years to develop in-vivo diagnosis methods such as cancerous cell targeting or even drugs. e-Proteins is working on new avidin derivatives that make targeting cancerous cells easier by limiting the occurrence of Avidin binding with healthy cells.
This new derivative plays two roles: (i) it targets cancerous cells, and (ii) it serves as a carrier for the active components of drugs having cancerous cells. This double function allows complete coherence and continuity between the diagnosis and the cure.
Molecular Weight
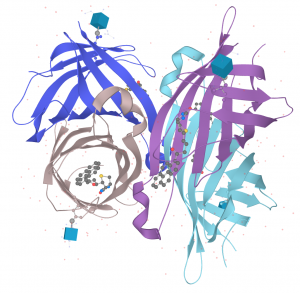
Avidin is a 66-KDa glycoprotein, produces in a hen’s oviduct and deposited in its egg albumen. Avidin’s molecular weight may differ slightly depending on the details in the literature.
With 99,9% of purety , the e-proteins product fits for all applications including the pharmaceutacl applicaiton
Avidin – Biotin System
This egg protein, binds the water-soluble vitamin biotin (vitamin B7 or H) very tightly and with an outstanding dissociation constant of ~10-15 M. Once formed, the bond between biotin and Avidin rapidly forms, remains unaffected by extremes of temperature, organic solvents, other denaturing agents, and pH.
The stability and affinity of the protein for biotin have also been extensively investigated and characterized. It folds into a quaternary tetramer structure of 4 identical amino acid compositions and sequence subunits containing 9 lysine residues.
Researchers extensively use the avidin-biotin system as a mediator in various biological applications, including isolation, localization, cytochemistry, immunoassay, and diagnostics. The alkaline properties and oligosaccharide content of the Avidin isolated from egg white – which at least appears to be responsible for some non-specific interaction at biological levels – had originally given the preference to using the bacterial biotin-binding analog, i.e., Streptavidin.
The bioengineers have corrected the non-specific absorption properties of Avidin to a large extent by developing a neutral and deglycosylated form called NeutraLite Avidin. This conjugate exhibits very low levels of non-specific absorption to biological materials and leaves many lysine residues available for chemical derivatization and conjugation.
How Avidin Binds the Biotin?
The biotin-binding site is a deep, pear-shaped pocket whose volume as well as the three-dimensional structure and orientation of the residues participating in hydrogen bonding with the vitamin are predetermined as complementary to that of the incoming vitamin. In the absence of biotin, the biotin-binding pocket contains five molecules of water that mimic the biotin (vitamin B7) structure in the binding site until biotin is in the bounding condition.
In the apo-protein (without ligand), the vitamin-binding pocket is fairly open due to the flexible L3,4-loop, thus allowing fast access of biotin to the binding site.
The β-barrel structured protein binds and buries biotin inside its central pocket, with the vitamin’s bicyclic ring located at the bottom of the cavity. Conformational readjustments of the protein, mainly involving the stiffened L3,4-loop, but also L5,6, trap the vitamin. During the process, three amino acid residues of L3,4 contribute additional interactions with biotin. Therefore, the extremely slow dissociation rate of biotin from Avidin arises from its high affinity for the protein.
How can Biotin Significantly Interfere with Lab Test ?
Identifying samples that already contain biotin technology can be difficult for assays using the biotin technology based on avidin. Indeed, patients taking biotin as a supplement could present high levels of biotins in their specimens. So, if the patient takes up to 300 mg per day of biotin (Vitamin B7), the concentration of biotin in their specimens can reach 1200ng/mL.
This presence of biotin in the specimens can cause significant interference with the lab test or with the diagnostic tests using the biotin technology such as avidin.
For the time being, the main criteria to define is whether the test can be affected by the biotin in the specimens based on the biotin concentration. This biotin level is set to 1200ng/mL.
FDA is investigating with all stakeholders to understand the interference of the biotin in the specimens with the test lab or diagnostics tests using the biotin technology.
What is the Avidin Market ?
The egg protein has usage in diagnostic kits, cell cultures to block biotin and pharmaceutical. All of those markets have their certification and quality requirements. e-Proteins is able to reach all of those certifications, including the virus inactivation when required.
What is the Avidin Purification Process ?
At e-Proteins, the purification process has been based on proven technology for more than 30 years. It was developed by Belovo Chemicals (Bastogne, Belgium) in the 1990s, a world wild egg technology leader. In 2012 e-Proteins launched an R&D project to improve and optimize this process to offer its customer the highest quality. Today our product presents a >99% purity and can be produced with an endotoxin-free certificate (batch size 50 gr).

25 tons of albumen , 800 000 eggs , are required to produce 1 kg of purified avidin
In 25,000 kg (25 tons) of albumen from 800,000 eggs, 1.2 kg of Avidin is available for extraction at 100% yield. The current yield is about 30%, so a production batch is about 500 gr of freeze-dried product.
The purifiaction step needs a dedicated resin with advanced binding capacity
What is the Iso-Electric Point of Avidin?
Avidin is highly cationic with an isoelectric point (pI) of about 10.5. Glycosylation occurs at the Asn-17 residue in a typical NXT(S) or Asn-Xxx-Thr(Ser) type carbohydrate-containing consensus sequence. Oligosaccharide components (heterogeneous structures composed largely of mannose (Man) and N-acetylglucosamine (GlcNac)) and positively charged amino acid residues (Lys & Arg) can interact nonspecifically with lectins and negatively charged cell surfaces and nucleic acids, thereby potentially causing nonspecific bindings in diagnostic and therapeutic applications1.
An avidin derivative enables us to avoid this non-specific bonding.
Bioengineering methods have been developed to suppress such nonspecific bindings. The result: NeutraLite Avidin, a nonspecific binding-free form of native protein, in which the signal/background ratio can be customarily adjusted (see Ultracoat70 or Oligomerized neutralite avidin) and the biotin-specific binding can be made reversible (see Nitroavidin, Avidin Monomer, Iminobiotin) to accommodate an ever larger range of applications.
Specifications
Avidin Properties
| Physico / chemistry | AVI90 | AVI100 | AVI100-EF |
|---|---|---|---|
| Purity | 90% | 100% | 100% |
| Activity
(U/mg) | > 11.0 | > 14.5 | > 14.5 |
| Moisture content
(% w : w) | < 5.0 | ||
| Ash (% w : w) | < 3.0 | < 0.5 | < 0.5 |
| Protein purity
(homogenous) | N/A | OK | OK |
| ID test
(no unspecified bands on SDS PAGE) | N/A | OK | OK |
o
| Microbiology | AVI90 | AVI100 | AVI100-EF |
|---|---|---|---|
| LPS Endotoxins
(IU/mg) | N/A | N/A | < 1,5 |
| Total Bacterial Count in 1 mg | <1 | < 1 | < 1 |
o
| Other | AVI90 | AVI100 | AVI100-EF |
|---|---|---|---|
| Appearance | white free flowing lyophilised powder, free of any visible impurities |
||
| Definition of activity | One Unit activity binds 1 µg of biotin | ||
| Country of Manufacturing | Belgium | ||
| Origin | Egg proteins from hens | ||
Discovery history
Details
The protein has discovered by Esmond Emerson Snell (1914–2003) but had not been isolated. In 1941, in collaboration with Paul Gyorgy, a biotin (Vitamin H) specialist, they confirmed that the isolated egg protein was the cause of biotin deficiency or “egg white injury”. The researchers at the University of Texas named the protein: avidalbumin representing the concatenation of Avid(= hungry) + albumin.
A few years later, the protein was renamed “Avidin” representing the concatenation of avid + biotin.